Abstract
Brain tumor segmentation from Magnetic Resonance Imaging scans is vital for both the diagnosis and treatment of brain cancers. It is widely accepted that accurate segmentation depends on multi-level information. However, exiting deep architectures for brain tumor segmentation fail to explicitly encourage the models to learn high-quality hierarchical features. In this paper, we propose a series of approaches to enhance the quality of the learnt hierarchical features. Our contributions incorporate four aspects. First, we extend the popular DeepMedic model to Multi-Level DeepMedic to make use of multi-level information for more accurate segmentation. Second, we propose a novel dual-force training scheme to promote the quality of multi-level features learnt from deep models. It is a general training scheme and can be applied to many exiting architectures, e.g., DeepMedic and U-Net. Third, we design a label distribution-based loss function as an auxiliary classifier to encourage the high-level layers of deep models to learn more abstract information. Finally, we propose a novel Multi-Layer Perceptron-based post-processing approach to refine the prediction results of deep models. Extensive experiments are conducted on two most recent brain tumor segmentation datasets, i.e., BRATS 2017 and BRATS 2015 datasets. Results on the two databases indicate that the proposed approaches consistently promote the segmentation performance of the two popular deep models.
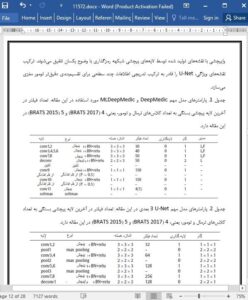
5. Conclusion
Accurate brain tumor segmentation depends on multi-level information. However, existing deep models do not explicitly guarantee the quality of the learnt hierarchical features. In this paper, we propose a dual-force training strategy to explicitly encourage deep models to learn high-quality multi-level features. This is realized by a label distribution-based loss function to learn the abstract semantic information and a softmax loss function for segmentation using multi-level features. The dual-force training strategy can be applied to many popular networks, e.g., DeepMedic and U-Net. Applying the proposed strategy to deep models only slightly increases the time and space complexity while training. Besides, we also propose an MLP-based post-processing method that can automatically learn post-processing rules from data rather than manual summarization. Extensive experiments on two most recent brain tumor segmentation databases justify the efficiency and effectiveness of the proposed approaches. One shortage of the MLP-based post processing method is that its training process is separated from that of DFN; therefore, the entire framework is not completely end-to-end. In the future, we will further enhance the segmentation capability of the deep architecture, so that a separate post-processing stage can be skipped.