Ti-8 Al-1 Mo-1 V was tested in solvents containing chloride, bromide or iodide ions. It was found that the region II plateau velocity could be correlated with the inverse of the viscosity for several solvents. It seems most likely that this reflects the influence of the viscosity on the diffusion coefficient of some species involved in a mass transfer process which limits the crack growth. Associated tests were performed in which the potential, pH and concentration of Cl− were varied, in some of the same solutions as used for the viscosity tests. It is concluded from these tests that theabsence of cathodic protection against cracking in solutions of low pH arises from an ohmic effect which isolates the crack tip from external potential control.
1. Introduction
Stress corrosion cracking (SCC) of titanium alloys, or any material, is a complex subject - for example, the scale (size) that may be examined ranges from the failure of large structures to atomistic descriptions of events at a propagating crack tip. In recent comprehensive reviews [1, 2] the range of the subject was demonstrated and it was shown that the myriad variables render any really complete and quantitative description of SCC unlikely at least in the near future. Thus, in selecting any aspect of the problem the constraints on experimental and theoretical analyses emerging from the investigation must be realized. The work which is described in this paper is an attempt to explain the controlling factors that determine the plateau velocity of a stress corrosion crack in some systems. The origin of this problem can be understood by examination of Fig. 1 which illustrates the extensive range of plateau velocities which may be observed in one alloy tested in various environments. It is noted that these environments contain liquids and gases, and in fact, if results for cadmium embrittlement [3] were present, could also include solid environments. Contemplation of Fig. 1 raised the obvious question of the factor(s) that control the plateau velocity and the point was selected as being worthy of further study. It was clear that one single physical property could not form the basis of an explanation of growth velocity - for example, it is difficult to see the connection (if any) between liquid mercury, molten halide salts and hydrogen gas. Thus, an initial restriction was placed on the range of these experiments and this paper only considers cracking in liquid environments.
6. Conclusions
For the conditions investigated, i.e. Ti-8 AI-1 Mo-1 V (820~ WQ) tested in solvents containing chloride, bromide or iodide ions, it was established experimentally that:
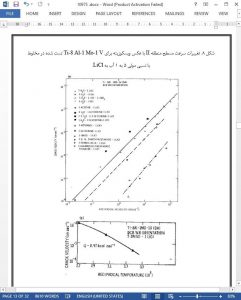
1. the region II plateau velocity varies inversely with the viscosity;
2. the activation energy for the region II plateau velocity shows the same temperature dependence as that for viscosity;
3. the plateau velocity in the several solvents may be correlated with the solution conductivity;
4. mixing the solvents gives non-additive results both on a viscosity and a conductivity basis.