Abstract
Polyaniline (PANI) and its nanocomposites containing TiO2, Ag, and Zn were electrocoated on an Al1050 electrode by cyclic voltammetry. The modified polymer and the nanocomposite films were characterized by cyclic voltammetry (CV), ultraviolet–visible spectrophotometry (UV–vis), Fourier-transform infrared spectroscopy-attenuated total reflectance (FTIR-ATR), scanning electron microscopy (SEM), energy-dispersion X-ray analysis (EDX), optical microscopy and electrochemical impedance spectroscopic (EIS) methods. The corrosion behavior of the PANI, PANI/TiO2, PANI/Ag and PANI/Zn nanocomposite films on an Al1050 electrode was studied in a 3.5% NaCl solution. The comparison results were obtained by applying Tafel extrapolation and EIS techniques. The findings indicate that PANI/Ag nanocomposite films yielded higher protection efficiency (PE = 97.54%) compared to PANI (PE = 91.41%), PANI/TiO2 (PE = 91.91%), and PANI/Zn (PE = 92.52%) nanocomposite films. The results show that the addition of nano-materials (TiO2, Ag and Zn) into the polymer matrix of PANI enhanced the electrical conductivity of the PANI/Zn film and the corrosion resistance of the polyaniline polymer. These findings were further confirmed by decreasing the oxygen and water permeability and increasing coating adhesion in the presence of TiO2, Ag and Zn nanomaterials in the PANI. The EIS measurements indicated that the incorporation of TiO2, Ag, and Zn into the coating increased both the charge transfer and pore resistance.
1. Introduction
Conducting polymers such as polyaniline (PANI), polypyrrole (PPy) or polythiophene (PTh) are used in industrial applications suchas rechargeable batteries [1], sensors [2], andcorrosionprotection [3–5]. Polyaniline (PANI)is a conducting polymer that has been widely studied, as it is believed that it has potential for electronic and optical applications due to its simple and reversible doping/dedoping chemistry, which enables control of several properties, such as electrical conductivity,free-volume and optical activity [6,7]. The charges stored in the polymer layer can be irreversibly consumed during the system’s redox reactions, and the ability of the corrosion protection with PANI may be lost over time [8].
4. Conclusions
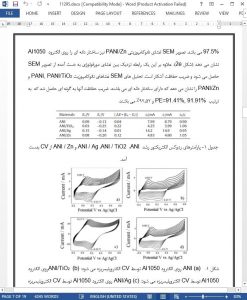
In this study, PANI, PANI/Zn, PANI/TiO2 and PANI/Agnanocomposite coatings were electropolymerized on an Al1050 substrate in a 0.5 M H2SO4 aqueous solution containing 0.4 M aniline and 1% nanoparticles under ultrasonic irradiation. The CV method was successfully employed for the preparation of TiO2, Ag and Zn nanoparticles in the presence of PANI. The modified electrodes were characterized by FTIR-ATR, SEM-EDX, EIS, and Tafel extrapolation methods. PANI, PANI/Zn, PANI/TiO2 and PANI/Ag-nanocomposites on Al1050 were tested for corrosion protection ability against a 3.5% NaCl solution. The highest protection efficiency was obtained for the PANI/Ag nanocomposite film (PE = 97.54%). Based on the corrosion test results, it can be concluded that the CV, SEM and EIS findings also supported the Tafel extrapolation results and thus confirmed that PANI/Ag nanocomposite films had the highest protective properties. Thus, we posit that PANI/Ag nanocomposite films might be used as protection materials for battery and supercapacitor device applications which are promising power generation technologies.