Abstract
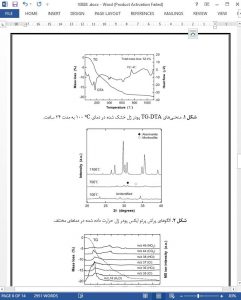
The thermal and biological properties of diopside, CaMgSi2O6 prepared by a sol–gel process using a metal alkoxide and metal salts were investigated for its applicability as a biomaterial. Precursor wet gel was synthesized by hydrolyzing a homogeneous solution consisting of Ca(NO3)2·4H2O, MgCl2·6H2O and Si(OC2H5)4 dissolved in ethanol. The effect of thermal treatment on crystallization of the hydrolyzed product was examined by DTA, XRD and TG–MS measurements. The dried gel powder was X-ray amorphous and crystallized into diopside single phase at 751.4 ◦C. This crystallization temperature was lower than that for the dried gel powder prepared by a sol–gel process using metal alkoxides reported by Nonami et al., which suggests that acidic compounds such as HNO3 and HCl generated in the powder during the heating process promotes the crystallization. Moreover, when the sintered body of diopside was immersed in simulated body fluid, an apatite layer was formed on the surface. Thus, the diopside prepared by the sol–gel process using the metal alkoxide and the metal salts without acidic catalysts addition was found to have an apatite-forming ability.
1. Introduction
Diopside (CaMgSi2O6), one of the pyroxene minerals, is known as an excellent bioactive material and has been the subject of many studies [1–8]. According to these studies, when diopside is immersed in simulated body fluid (SBF) apatite-like calcium phosphates are formed on its surface, giving a good bioactivity [9,10]. In addition, the sintered body of diopside seems to bond to living bone tissues more rapidly than apatite [11,12]. Moreover, diopside has a fairly high mechanical strength and superb biological affinity [13–15]. Diopside is therefore considered to have a potential as a biomaterial for artificial bone and tooth.
4. Conclusions
Diopside was prepared by a sol–gel process using a metal alkoxide and metal salts as the starting materials, and the effect of thermal treatment on crystallization of the dried gel powder and the bioactivity of the sintered body of diopside were examined by means of the immersion of the diopside in simulated body fluid (SBF). Referring to DTA and XRD measurements, the dried gel powder prepared by this method was suggested to crystallize into diopside single phase at 751.4 ◦C. The resultant powder possessed a higher crystallinity than that produced by the alkoxide method. Acidic compounds such as HNO3 and HCl generated in the powder during the heating process promoted the crystallization in thermal treatment. In the evaluation of the bioactivity, an apatite layer was found to be formed on the surface of diopside in SBF. This result suggested that the sintered body of diopside prepared by the sol–gel process using the metal alkoxide and the metal salts without acidic catalysts addition has an apatite-forming ability and a remarkable bioactivity.