Abstract
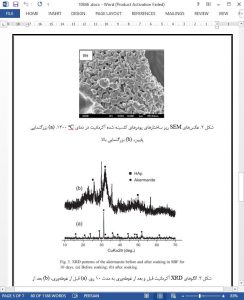
Pure akermanite (Ca2MgSi2O7) powders were synthesized by sol – gel method. The akermanite powders were composed of polycrystalline particles with dimensions of 5 – 40 Am. The apatite-formation ability of the akermanite was examined by soaking it in a simulated body fluid (SBF), and the result showed that hydroxyapatite (HAp) was formed after soaking for 10 days. Our study indicated that akermanite possessed apatite-formation ability and might be used for preparation of new biomaterials.
1. Introduction
Previous studies have shown that some glasses and glassceramics containing Si, Ca and Mg were highly bioactive and could be used for biomedical applications [1– 3]. Furthermore, diopside (CaMgSi2O6), a Si, Ca and Mg containing ceramic, has been reported to be bioactive and closely bonded to bone tissue when implanted in rabbits [4 – 7]. As an analogue with diopside in component, akermanite (Ca2MgSi2O7) is also a Si-, Ca- and Mg-containing ceramic. Therefore, it is reasonable to assume that akermanite may be a bioactive material. Akermanite is a mineral, with a melting temperature of >1450 jC and a density of 2.944 g/cm3 , and so far has no important industrial applications. The naturally occurring akermanite was often not pure and associated with other minerals, and to our knowledge, there was no report about chemical synthesis of pure akermanite.
4. Conclusions
In summary, pure akermanite powders were synthesized by sol – gel method and calcination at 1300 jC, and the size of akermanite powders was about 5 –40 Am. The in vitro study showed that powders could induce hydroxylapatite formation on their surface after 10 days of soaking in SBF. Our study suggests that akermanite possessed apatite-formation ability and might be a potential candidate as tissue repair biomaterials.