Abstract
In order to construct micelles with desirable characteristics and predictable behaviour, insights in micellization mechanisms and factors leading to micelle stabilization are necessary.
In this study, Brij S10, Brij S20, Tween 20, Tween 80 and their binary systems were examined. Spectrofluorimetry was used in order to obtain experimental values of critical micelle concentrations from which thermodynamic parameters were calculated.
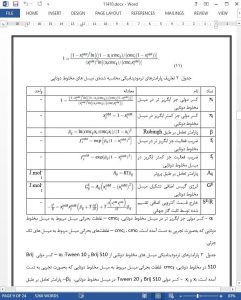
The aim of the study was to determine the nature of the excess Gibbs energy in examined systems. Analysis of the function β = f(T), allowed us to define how variations in surfactants’ structure affect the thermodynamic stabilization of binary systems.
Results show that the difference in the length of hydrophobic segments in mixed micelles is responsible for the additional stabilization of Brij S10/Tween 20 and Brij S20/Tween 20 binary systems. In comparison to them, mixed micelles containing alkyl chains of the same length have lower excess entropy values. Introduction of olefin bond in surfactant’s alkyl chain (Tween 80) destabilizes the binary system and reduces its excess entropy values. Elongation of hydrophilic segments results in a favourable enthalpic and an unfavourable entropic contribution to the excess Gibbs energy.
1. Introduction
Understanding of the micellization process is necessary in order to expand the application area of surfactants. In some cases, surfactant molecules, when combined in solutions, build mixed micelles that are thermodynamically more stable than single-component micelles of individual surfactants. Such binary systems are characterized with lower critical micelle concentrations (cmc) than pure solutions of surfactants [1–6]. Changes that lead to the stabilization of a mixed micelle need to be explored in order to construct new types of mixed micelles, predict their behaviour and facilitate their safe application industry-wide [7,8].
5. Conclusions
Regular solution theory cannot be applied on investigated binary mixtures without certain corrections regarding the existence of the excess entropy. Changes in the values of the b interaction parameter for analysed binary systems can be described as a function of temperature. This is a conformation of both entropic and enthalpic nature of excess Gibbs energy of micellization. The difference in length of the hydrophobic chains in mixed micelles (present in Brij S10/Tween 20 and Brij S20/Tween 20 micelles) is responsible for their additional stabilization. In comparison to them, systems built of monomers with alkyl chains of the same length have lower excess entropy values. Since longer polyoxyethylene chains engage in more hydrogen bonds with solvent molecules, elongation of polar segments results in favourable enthalpic contribution that opposes an unfavourable entropic contribution. Olefin bond present in Tween 80 molecules is responsible for an increase in the area of hydrophobic surface of a micelle’s core that needs to be hydrated. This effect reduces both enthalpic and entropic input in excess Gibbs energy.