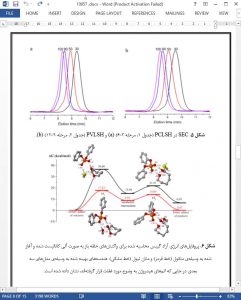
A novel metal-free and protecting-group-free synthesis method to prepare telechelic thiolfunctionalized polyesters is developed by employing organocatalysis. A scope of Brønsted acids, including trifuoromethanesulfonic acid (1), HCl.Et2O (2), diphenyl phosphate (3), γ-resorcylic acid (4) and methanesulfonic acid (5), are evaluated to promote ring-opening polymerization of ε-caprolactone with unprotected 6-mercapto-1-hexanol as the multifunctional initiator. Among them, diphenyl phosphate (3) exhibits great chemoselectivity and efciency, which allows for simply synthesis of thiol-terminated poly(ε-caprolactone) with near-quantitative thiol fdelity, full monomer conversion, controlled molecular weight and narrow polydispersity. Kinetic study confrms living/controlled nature of the organocatalyzed chemoselective polymerizations. Density functional theory calculation illustrates that the chemoselectivity of diphenyl phosphate (3) is attributed to the stronger bifunctional activation of monomer and initiator/chain-end as well as the lower energy in hydroxyl pathway than thiol one. Moreover, series of tailor-made telechelic thiol-terminated poly(δ-valerolactone) and block copolymers are efciently generated under mild conditions.
Organocatalysis has been deeply investigated and widely applied in the chemical transformations1 . Numerous excellent contributions were reported in this blooming research area2–8 . In polymer chemistry, organocatalysis provided remarkable opportunities in precision well-defned polymers9–11. Te features of the use of small organic molecules as the catalyst or initiator in ring-opening polymerization (ROP) of cyclic monomers were explored by many groups12–16. Te classes of organocatalysts have been continuously developed based on the general polymerization mechanisms of electrophilic monomer activation, nucleophilic monomer activation, initiator or chain-end activation and bifunctional activation of monomer and initiator/chain end17,18. Despite tremendous progress was made, bottlenecks still remained in organocatalyzed ROP, such as chemoselectivity, stereoselectivity and switchable catalysis18,19.
Characterizations
NMR spectra were recorded on a Bruker (400MHz) in CDCl3 with tetramethylsilane (TMS) as the internal reference. Size exclusion chromatography (SEC) was performed on Wyatt system equipped with a SSI 1500 pump and a Waters Styragel HR 2.5 μm, 300 mm ×7.8 mm column by using THF (0.7 mLmin−1 ) as eluent at room temperature. Matrix assisted laser desorption ionization time of fight mass spectra (MALDI TOF MS) were recorded at 25 kV on the Bruker mass spectrometer (ultraextreme). Te polymer and the matrix 2,5-dihydroxybenzoic acid (DHB) were dissolved in CH2Cl2. 1 μl of the sample solution was piped onto the thin NaI crystal layer and dried in air. All mass spectra were collected by employing 500 individual laser shots. Transmission electronic microscopy (TEM) was conducted on a JEM-200cx operating at 200 kV. Te sample was prepared by dipping the TEM copper grid to a dilute dispersion of silver nanoparticles in chloroform and solvent was evaporated at room temperature.