Abstract
Background The incidence of chronic disease is elevated in women after menopause. Natural variation in muscle expression of the estrogen receptor (ER)α is inversely associated with plasma insulin and adiposity. Moreover, reduced muscle ERα expression levels are observed in women and animals presenting clinical features of the metabolic syndrome (MetSyn). Considering that metabolic dysfunction impacts nearly a quarter of the U.S. adult population and elevates chronic disease risk including type 2 diabetes, heart disease, and certain cancers, treatment strategies to combat metabolic dysfunction and associated pathologies are desperately needed.
Scope of the review This review will provide evidence supporting a critical and protective role for skeletal muscle ERα in the regulation of metabolic homeostasis and insulin sensitivity, and propose novel ERα targets involved in the maintenance of metabolic health.
Major conclusions Studies identifying ERα-regulated pathways essential for disease prevention will lay the important foundation for the rational design of novel therapeutics to improve the metabolic health of women while limiting secondary complications that have plagued traditional hormone replacement interventions.
1. INTRODUCTION
For over two decades researchers have shown strong relationships between estrogen action and metabolic health in women. Moreover, epidemiological reports indicate that chronic disease incidence increases in women following menopause. Considering that menopause occurs on average by age 51 (National Institutes of Health, NIA www. nia.nih.gov), and that life expectancy has increased for white females to w80.6 years (The National Vital Statistics Report, 2012), women in the modern era are challenged with heightened disease risk associated with increasing adiposity and metabolic dysfunction for up to three decades of life. Although many researchers and clinicians have focused on the impact of replacement estrogens to ameliorate clinical symptoms and provide protective health benefit, an incomplete understanding of hormone action as well as estrogen receptor distribution and function has contributed to our continued confusion and failure to advance therapeutic strategies to combat chronic diseaseassociated pathologies for women.
8. CONCLUSIONS AND PERSPECTIVES
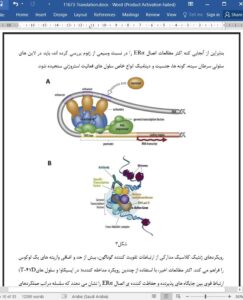
In recent years, novel molecular targets have emerged offering the prospect of pharmacological intervention to restore metabolic homeostasis and insulin action, as well as ameliorate complications associated with type 2 diabetes and obesity. The inherent beauty of targeting ERa therapeutically is underscored by decades of research and in depth knowledge related to biological/clinical efficacy and toxicity profiles obtained for estradiol/SERMs during preclinical studies in animal models and clinical trials in human subjects. Although estrogen treatment (primarily 17b-estradiol) is shown to promote energy homeostasis, improve body fat distribution, and diminish insulin resistance, b-cell dysfunction and inflammation, the challenge with chronic hormone administration is the narrow therapeutic index. Thus, the translation of the basic advances in diabetes and obesity treatment described in this review, although successful in rodents, is problematic when extending to clinical practice. However, ten years after the WHI concluded that the risks of hormone therapy outweighed its benefits, reevaluation of the WHI findings and determination that the risks of breast cancer, coronary heart disease, stroke, and pulmonary embolism with estrogen-progestin treatment were overstated, prompted a position statement by the North American Menopause Society stating that HRT has a role in short-term treatment of menopausal symptoms [167]. Thus, considering the new and more positive light estradiol is receiving by clinical experts, it will be important to determine whether short-term treatment with a well-designed ERa agonist during early menopause offers protection against metabolic dysfunction and insulin resistance.