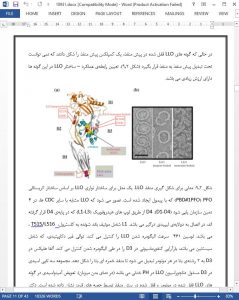
Abstract
The cholesterol-dependent cytolysins (CDCs) are a large family of pore-forming toxins that are produced by numerous Gram-positive bacterial pathogens. These toxins are released in the extracellular environment as water-soluble monomers or dimers that bind to cholesterol-rich membranes and assemble into large pore complexes. Depending upon their concentration, the nature of the host cell and membrane (cytoplasmic or intracellular) they target, the CDCs can elicit many different cellular responses. Among the CDCs, listeriolysin O (LLO), which is a major virulence factor of the facultative intracellular pathogen Listeria monocytogenes, is involved in several stages of the intracellular lifecycle of the bacterium and displays unique characteristics. It has long been known that following L. monocytogenes internalization into host cells, LLO disrupts the internalization vacuole, enabling the bacterium to replicate into the host cell cytosol. LLO is then used by cytosolic bacteria to spread from cell to cell, avoiding bacterial exposure to the extracellular environment. Although LLO is continuously produced during the intracellular lifecycle of L. monocytogenes, several processes limit its toxicity to ensure the survival of infected cells. It was previously thought that LLO activity was limited to mediating vacuolar escape during bacterial entry and cell to cell spreading. This concept has been challenged by compelling evidence suggesting that LLO secreted by extracellular L. monocytogenes perforates the host cell plasma membrane, triggering important host cell responses. This chapter provides an overview of the well-established intracellular activity of LLO and the multiple roles attributed to LLO secreted by extracellular L. monocytogenes.
Listeriolysin O is a Major Virulence Factor of L. monocytogenes Listeriosis
The Gram-positive, facultative anaerobe Listeria monocytogenes is the causative agent of listeriosis, a life-threatening disease associated with a very high rate of mortality in humans (20–30 %) and numerous other vertebrate species [1, 2]. This bacterium was isolated from diseased rabbits in 1926 by E. G. D. Murray and was recognized as the cause of a severe human foodborne illness in the early 1980s [3–5]. L. monocytogenes is ubiquitous in the environment, where it is found in soils, water, and plants, and frequently contaminates a large variety of raw and processed foods. The versatility of this organism comes from its ability to grow at a wide range of temperatures (1–45 °C) and pH (4.4–9.6), at high concentrations of salts (up to 10 % NaCl), and to resist the harsh environment of the animal gut [6–9].
Concluding Remarks
LLO has emerged as a multifunctional toxin that regulates the intracellular life-cycle of L. monocytogenes in diverse cell types and affects the innate and adaptive immune responses. Many more studies are necessary to better delineate the mechanisms of action of this toxin. In particular, how LLO facilitates the disruption of the endocytic vacuoles containing L. monocytogenes is still unclear. LLO secreted by extracellular bacteria also targets and affects the biology of multiple cell types, from epithelial cells to T lymphocytes. Extracellular LLO may control several stages of the L. monocytogenes intracellular lifecycle including bacterial internalization, vacuolar escape, and efficient intracellular replication. Extracellular LLO may also affect many cell types that the bacterium does not infect. For example, LLO can modulate the production of inflammatory messengers and cell death pathways independently of host cell invasion. A major challenge in the field is now to distinguish the extracellular from intracellular activities of LLO in vivo, and to determine which, if not all, of the host cell responses to LLO observed in cell culture models play a substantial role during pathogenesis. A fascinating aspect of LLO is its dual role during infection: whereas LLO is indispensable for pathogenesis, it is a major immunogenic molecule that ultimately elicits Listeria monocytogenes killing by the immunocompetent host. Due to its unique set of properties, LLO has emerged as a promising tool for the development of vaccine adjuvants. However, the molecular basis of the LLO immunomodulatory activity is poorly understood. Gaining knowledge on the interplay between LLO and host cells will importantly benefit basic research on pathogen-host interaction, pore-forming toxins, immunity, as well as the design of vaccines.